Insights+: EMA Marketing Authorization of New Drugs in August 2023
Shots:
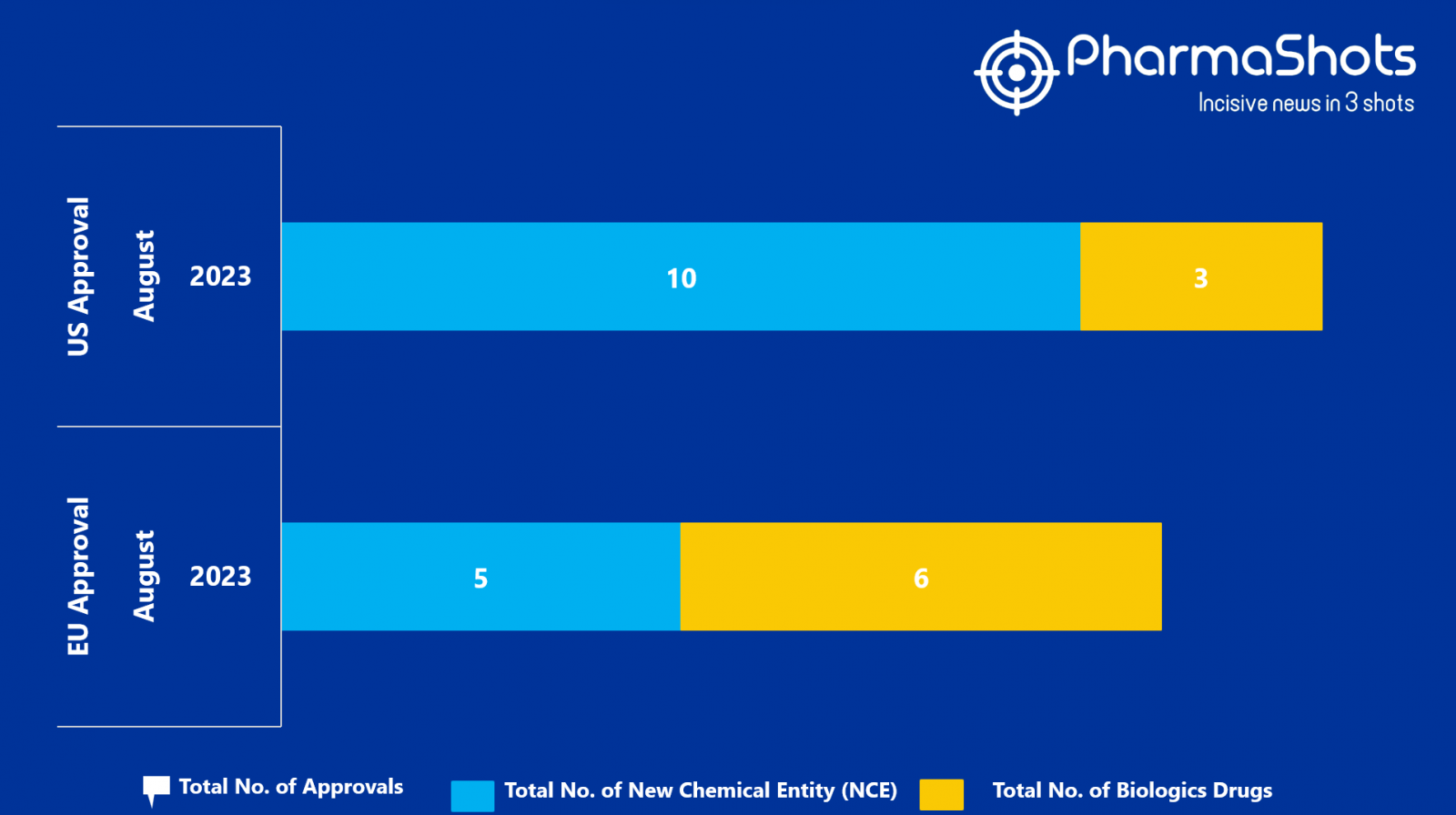
- The EMA approved 5 New Chemical Entity (NCE) and 6 Biologic Drugs in August 2023, leading to treatments for patients and advances in the healthcare industry
- In August 2023, the major highlight drugs were Aquipta (atogepant) approved for the preventive treatment of migraine in adults & Opdivo (nivolumab) as an adjuvant treatment for completely resected stage IIB or IIC melanoma
- PharmaShots has compiled a list of a total of 11 new drugs approved by the EMA in August 2023
Lonsurf
Active ingredient: trifluridine and tipiracil Approved: Aug 01, 2023
Company: Taiho Pharmaceutical Disease: Colorectal Cancer
- The EC has approved Lonsurf + bevacizumab for adult patients with mCRC who received 2 prior anti-cancer treatments incl. fluoropyrimidine-, oxaliplatin & irinotecan-based chemotherapies, anti-VEGF agents, and/or anti-EGFR agents. The trial was conducted by Servier & Taiho Oncology
- The approval was based on the P-III trial (SUNLIGHT) evaluating trifluridine/tipiracil + bevacizumab vs trifluridine/tipiracil alone in a ratio (1:1) in 492 patients. The primary objective was to evaluate trifluridine/tipiracil + bevacizumab in terms of OS & 2EPs were PFS, ORR, DCR & QoL, safety & tolerability
- The marketing authorization is valid in 27 EU countries, Iceland, Northern Ireland, Liechtenstein & Norway. Lonsurf, an oral nucleoside antitumor agent discovered & developed by Taiho Pharmaceutical
Aquipta
Active ingredient: atogepant Approved: Aug 17, 2023
Company: AbbVie Disease: Migraine
- The EC has approved Aquipta (CGRP receptor antagonist) for the prophylaxis of migraine in adults who have ≥4 migraine days per month. The approval was based on the 2 P-III studies (PROGRESS) & (ADVANCE) evaluating Aquipta (60mg, qd) vs PBO in 778 & 910 patients
- Both studies met their 1EPs i.e., reduction in mean monthly migraine days vs PBO, improvements were observed in all 2EPs with Aquipta (60mg, qd). In the (PROGRESS) & (ADVANCE) study, a reduction in the changes from baseline in MMDs of 6.8 vs 5.1 days & 4.1 vs 2.5 days; 40% vs 27% & 59% vs 29% achieved a 50% reduction in MMDs, respectively
- The therapy was approved in the US for chronic & episodic migraines and in Canada for episodic migraines under the brand name Qulipta
Tecvayli
Active ingredient: teclistamab Approved: Aug 18, 2023
Company: Janssen Disease: Multiple Myeloma
- The EC has granted conditional marketing authorization to Talvey as a monotx. for adult patients with RRMM who received 3 prior therapies
- The authorization was based on the P-I/II study (MonumenTAL-1) evaluating talquetamab, which showed ORR across 0.8mg/kg, q2w, and 0.4mg/kg, qw doses. 71.7% and 74.1% treated at 0.8mg/kg, q2w, and 0.4mg/kg, qw dose achieved a response with a median follow-up of 12.7 and 18.8mos, VGPR or better (60.8% and 59.5%) and CR (38.7% and 33.6%), respectively
- Responses were durable with m-DoR (not reached and 9.5mos.) in both doses; 76.3% and 51.5% maintained a response for 9mos., adverse reactions leading to treatment discontinuation due to ICANS (1.1%) & weight loss (0.9%). The results were presented at ASCO & EHA 2023
4. Iveric Bio Reports EMA Acceptance of MAA for Avacincaptad Pegol to Treat Geographic Atrophy
Izervay
Active ingredient: Avacincaptad Pegol Approved: Aug 18, 2023
Company: Iveric Bio Disease: Geographic Atrophy
- The EMA has accepted the MAA for regulatory review of avacincaptad pegol, a complement C5 inhibitor for the treatment of GA secondary to AMD. The MAA was based on the results from the P-III (GATHER1 & 2) trials evaluating safety and efficacy of ACP (qm, 2mg, IVT) in 286 & 448 patients
- The primary analysis showed a significant reduction in the rate of GA growth in patients treated with ACP over sham in each registrational trial over a 12mos. period while safety was evaluated in ~700 patients across both trials
- Avacincaptad pegol, a complement C5 protein inhibitor was approved in the US as Izervay for the treatment of GA secondary to AMD
REGN 1979
Active ingredient: Odronextamab Approved: Aug 18, 2023
Company: Regeneron Disease: Follicular Lymphoma and DLBCL
- The EMA has accepted the MAA for review of odronextamab (CD20xCD3 bispecific Ab) for adult patients with r/r FL or r/r DLBCL who have progressed after 2 prior systemic therapies. The MAA was based on the P-I study (ELM-1) and P-II trial (ELM-2) evaluating odronextamab in FL and DLBCL
- Both studies’ results presented at ASH for r/r FL showed ORR (82%) in patients with grades 1 to 3a disease, CR (75%), median duration of complete response (mDOCR) was 20.5mos., m-PFS (20mos.), m-OS (not reached)
- Among CAR-T naïve & post-CAR-T patients showed ORR (49% & 48%), CR (31% & 32%), mDOCR was 18mos. & not reached, respectively for r/r DLBCL. The company continues to advance odronextamab in the P-III development program for earlier lines of therapy & additional B-NHLs
Opdivo
Active ingredient: nivolumab Approved: Aug 23, 2023
Company: BMS Disease: Melanoma
- The EC has approved Opdivo as monotx. for the adjuvant treatment of adults & adolescents aged ≥12yrs. with stage IIB or IIC melanoma who have undergone complete resection
- The approval was based on the results from the P-III trial (CheckMate -76K) evaluating Opdivo (480mg, q4w for ~12mos.) vs PBO which showed a 58% reduction in risk of recurrence or death with a minimum follow-up of 7.8mos., 12mos. RFS rates were 89% vs 79%, RFS benefit was observed across predefined subgroups incl. T category and disease stage
- The safety profile was consistent with prior reported studies. Opdivo (PD-1 immune checkpoint inhibitor) is currently approved in 65+ countries, incl. the US, the EU, Japan, and China
Abrysvo
Active ingredient: RSVpreF Approved: Aug 25, 2023
Company: Pfizer Disease: Respiratory Syncytial Virus
- The EC has granted marketing authorization for Abrysvo, the RSVpreF vaccine for older adults & for immunization of pregnant individuals to help protect their infants immediately from birth through 6mos.
- The authorization was based on the P-III study (RENOIR) in adults aged ≥60yrs. & (MATISSE) trial in infants born to healthy individuals vaccinated during pregnancy evaluating the efficacy, immunogenicity & safety of Abrysvo
- In the (RENOIR) study, the RSVpreF vaccine prevented RSV-associated LRTD and RSV-associated acute respiratory illness in adults aged ≥60yrs. while the vaccine administered during pregnancy in (MATISSE) trial was effective in infants with no safety concerns. The authorization is valid in all 27 EU member states, Iceland, Liechtenstein & Norway
Evrysdi
Active ingredient: risdiplam Approved: Aug 29, 2023
Company: Roche Disease: Spinal Muscular Atrophy
- The EC has expanded the marketing authorization for Evrysdi to include infants with SMA Type 1, Type 2, or Type 3 or with one to four SMN2 copies from birth to ≤2mos.
- The approval was based on the interim analysis from the (RAINBOWFISH) study. All 6 infants incl. in the analysis were able to sit after 1yr. of treatment with Evrysdi i.e., 100% (6/6) while 67% (4/6) could stand, 50% (3/6) walked independently & all infants were alive at 12mos. without permanent ventilation
- The safety profile of Evrysdi in pre-symptomatic babies was consistent with the safety profile in prior trials. Evrysdi is currently being studied in the P-II/III trial (MANATEE) in combination with an anti-myostatin molecule targeting muscle growth for SMA
Keytruda
Active ingredient: pembrolizumab Approved: Aug 29, 2023
Company: Merck Disease: Gastric Cancer
- The EC has approved Merck’s Keytruda (anti-PD-1 therapy) in combination with trastuzumab, fluoropyrimidine- and Pt-containing CT as 1L treatment of LA unresectable or metastatic HER2+ gastric or GEJ adenocarcinoma in adults whose tumors express PD-L1
- The approval was based on results from the P-III trial (KEYNOTE-811) evaluating Keytruda (200mg, q3w) + trastuzumab and CT vs trastuzumab and CT alone in 732 patients which showed significant improvement in PFS, & ORR, ≥80% of patients had tumors that were PD-L1+
- The trend toward improvement in OS in the ITT population but these results did not meet statistical significance per the pre-specified statistical analysis plan. The results will be presented at ESMO 2023
Comirnaty Omicron XBB.1.5
Active ingredient: raxtozinameran Approved: Aug 30, 2023
Company: Pfizer and BioNTech Disease: COVID-19
- The EMA’s CHMP has recommended marketing authorization for Omicron XBB.1.5-adapted monovalent COVID-19 vaccine (Comirnaty Omicron XBB.1.5) as a single dose for individuals aged ≥5yrs.
- The recommendation was based on the previous clinical, non-clinical & real-world evidence of COVID-19 vaccines. The recommendation is also based on pre-clinical data showing that the COVID-19 vaccine generates an improved response against multiple XBB-related sublineages
- The committee also recommended the updated vaccine for children aged 6mos. to 4yrs. given as part of all of the primary three-dose vaccination series or as a single dose for individuals with a complete COVID-19 primary vaccination course. In the pre-clinical data, the updated vaccine induces serum Abs that neutralize the WHO-designated variant of interest EG.5.1 (Eris)
11. InflaRx Report EMA’s Validation of MAA for Vilobelimab to Treat Critically Ill COVID-19 Patients
Gohibic
Active ingredient: Vilobelimab Approved: Aug 30, 2023
Company: InflaRx Disease: COVID-19
- The EMA has validated the MAA for Vilobelimab to treat critically ill COVID-19 patients. The application is now under regulatory review by CHMP under the centralized procedure which valid to all 27 member states of the EU
- The submission was based on the P-III trial (PANAMO) trial evaluating vilobelimab in invasively mechanically ventilated COVID-19 patients in intensive care units which showed that vilobelimab improved survival with a relative reduction in 28-day all-cause mortality of 23.9%
- The results were published in The Lancet Respiratory Medicine. The company continues to discuss with the US FDA related to the submission of a BLA for full approval of Gohibic (vilobelimab)
Note: Omicron XBB.1.5-adapted COVID-19 Vaccine received EMA’s CHMP positive opinion; EC’s Marketing Authorization for Abrysvo & Evrysdi; EMA’s Validation of MAA for Vilobelimab; Type II variation application approval from EC for Tecvayli and EMA Acceptance of MAA for Avacincaptad Pegol & Odronextamab
Related Post: Insights+: EMA Marketing Authorization of New Drugs in July 2023




